Process Validation: The Complete Guide to Quality Assurance in Six Sigma
In today’s heavily regulated industries, process validation stands as a critical pillar of quality assurance.
From pharmaceutical manufacturing to medical device production, companies must prove their processes consistently deliver products that meet predetermined specifications.
Transform your quality management skills with proven Six Sigma methodologies
Learn how to implement rigorous process validation techniques in regulated industries.
Process validation isn’t just about checking boxes for regulators—it’s about building quality into products from the ground up.
When integrated with methodologies like Six Sigma certification, process validation becomes a powerful tool for ensuring consistent quality while minimizing waste and variation.
Key highlights
- Validation vs. verification differences explained
- Three stages of process validation
- Six Sigma tools for validation
- Regulatory requirements and compliance
What Is Process Validation?
Process validation forms the backbone of quality assurance in regulated industries. It’s not just a regulatory checkbox—it’s a systematic approach that ensures manufacturing processes consistently produce products meeting predetermined quality standards.
The FDA defines process validation as “the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product.“
This process validation definition emphasizes both scientific rigor and the need for consistency throughout the product lifecycle.
For pharmaceutical and medical device manufacturers, process validation isn’t optional. It’s mandated by regulatory bodies worldwide, including the FDA (21 CFR Parts 210 and 211), the European Medicines Agency, and other international authorities.
These regulations exist because validation directly impacts patient safety and product efficacy.
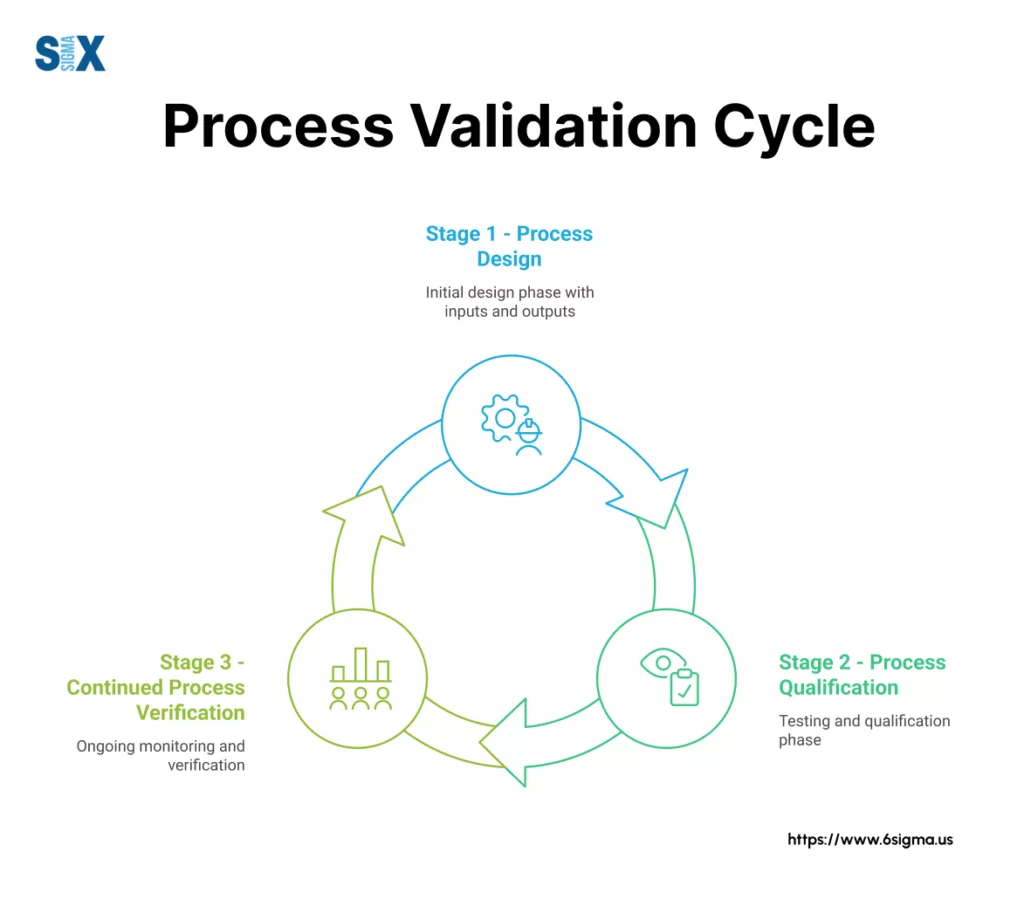
The regulatory framework for process validation has evolved significantly over the past decade. The FDA’s 2011 guidance document shifted the approach from a one-time validation event to a lifecycle concept with three distinct stages:
- Process Design: Building quality into the process through careful development and scale-up
- Process Qualification: Confirming the process design can perform effectively during commercial manufacturing
- Continued Process Verification: Maintaining the validated state through ongoing monitoring
This lifecycle approach aligns perfectly with modern quality management systems, particularly those influenced by lean introduction principles. Both emphasize building quality into processes rather than inspecting it into finished products.
Quality management systems benefit from process validation in several ways:
First, validation provides documented evidence that processes operate consistently within established parameters. This evidence becomes part of the quality system documentation, supporting both internal quality assurance and external regulatory inspections.
Second, the validation process identifies critical quality attributes and process parameters that must be controlled. These become key inputs for developing effective quality control strategies.
Third, validation establishes the foundation for continuous improvement initiatives. By thoroughly understanding process capabilities and limitations, teams can identify opportunities for optimization while maintaining validated status.
Many companies integrate process validation with broader quality approaches like Quality by Design (QbD).
This integration helps ensure validation activities focus on parameters that genuinely impact product quality rather than simply satisfying documentation requirements.
The Relationship Between Process Validation and Six Sigma
Process validation and Six Sigma might seem like separate quality approaches, but they actually complement each other perfectly.
Organizations that integrate these approaches gain a powerful advantage: validation activities become more efficient, data-driven, and focused on what truly matters for product quality.
This integration starts with understanding how validation fits within the DMAIC methodology.
The DMAIC framework from Six Sigma certification programs provides a structured approach to process validation:
Define: During this phase, validation teams identify critical quality attributes (CQAs) and critical process parameters (CPPs) that must be validated.
They establish validation acceptance criteria based on customer requirements and regulatory standards. The validation master plan often takes shape here, outlining the scope and approach for validation activities.
Measure: This phase focuses on developing measurement systems that can reliably collect validation data. Teams conduct measurement system analysis (MSA) to ensure test methods are capable of detecting meaningful variations.
Analyze: Here, teams examine relationships between process inputs and outputs to understand what drives process performance. This analysis helps identify which parameters must be tightly controlled during validation studies.
Improve: During this phase, teams optimize process parameters to enhance consistency before validation begins. Design of Experiments (DOE) techniques help determine optimal operating ranges for critical parameters. Process validation protocols are finalized based on these optimized conditions.
Control: This phase aligns perfectly with Stage 3 of process validation (Continued Process Verification). Teams implement control plans to maintain the validated state, including statistical process control (SPC) and routine monitoring of critical parameters.
The statistical approach to validation represents another key intersection between these methodologies. Six Sigma brings statistical rigor to validation activities through:
- Sample size determination based on statistical power
- Capability analysis to quantify process performance (Cp, Cpk)
- Statistical tolerance intervals to establish acceptance criteria
- Control charts to detect process shifts during validation runs
- Hypothesis testing to confirm process consistency
These statistical methods transform validation from a checkbox activity into a meaningful assessment of process capability. Rather than simply collecting data, validation teams can quantify the degree of confidence in their processes.
Quality metrics provide the third connection point between Six Sigma and process validation. Key metrics used in both approaches include:
- Process capability indices (Cp, Cpk) that measure how well a process meets specifications
- Right-first-time rates that track process consistency
- Defects per million opportunities (DPMO) that quantify quality levels
- Process sigma levels that indicate overall process performance
- Yield metrics that measure process efficiency
These metrics serve dual purposes: they demonstrate validated status to regulators while providing data for continuous improvement efforts.
Become a Process Validation Expert with Six Sigma Black Belt
Lead complex process improvement projects with advanced Six Sigma techniques
Three Stages of Process Validation in Six Sigma Projects
The FDA’s current approach to process validation follows a lifecycle model with three distinct stages.
Each stage builds upon the previous one, forming a continuous loop of design, qualification, and verification.
For Six Sigma practitioners, these stages provide natural integration points for DMAIC tools and statistical analysis. Let’s examine how each stage works within a Six Sigma context and the specific activities involved.
Process Design Phase
The Process Design phase lays the foundation for successful validation. During this stage, Six Sigma teams develop a process that can consistently deliver quality products at commercial scale.
Design considerations during this phase focus on translating product requirements into process parameters.
Teams identify Critical Quality Attributes (CQAs) that directly impact product performance and safety. They then determine which process parameters affect these attributes, designating them as Critical Process Parameters (CPPs).
Six Sigma tools like Quality Function Deployment (QFD) help connect product requirements to process specifications.
Design of Experiments (DOE) techniques allow teams to efficiently explore how different parameters interact and affect quality attributes.
Risk assessment plays a crucial role in this stage. Teams use tools like Failure Mode and Effects Analysis (FMEA) to identify potential failure points and prioritize control strategies. This risk-based approach ensures validation efforts focus on aspects most likely to impact product quality.
Root cause analysis techniques help teams understand underlying causes of variability, enabling them to design more robust processes. By addressing root causes early in development, companies avoid costly problems during commercial production.
Quality by Design (QbD) principles guide the entire Process Design phase. QbD emphasizes building quality into products through scientific understanding rather than testing quality into finished products.
Key QbD elements include:
- Defining a target product profile based on patient needs
- Identifying critical quality attributes that must be controlled
- Understanding how process parameters and material attributes affect these quality attributes
- Establishing a design space where quality is assured
- Implementing a control strategy based on risk management
The output of this stage includes process characterization reports, risk assessments, and a preliminary control strategy. These documents feed directly into the process validation protocol developed in the next stage.
Process Qualification Phase
The Process Qualification phase confirms that the process design can perform effectively during commercial manufacturing.
This stage encompasses both equipment qualification and process performance qualification.
Equipment qualification follows the traditional IQ/OQ/PQ approach:
Installation Qualification (IQ) verifies that equipment is properly installed according to specifications. This includes checking physical installation, utility connections, calibration status, and documentation.
Six Sigma teams often use detailed checklists to ensure nothing is overlooked.
Operational Qualification (OQ) demonstrates that equipment operates within established parameters. This involves challenging equipment under normal and stress conditions to verify proper functioning. Statistical analysis helps determine appropriate test conditions and acceptance criteria.
Performance Qualification (PQ) confirms that equipment consistently performs as intended within the process.
This often involves running the process with actual materials under normal operating conditions. Six Sigma tools like capability analysis (Cp/Cpk) quantify how well the equipment performs against specifications.
Manufacturing process validation builds upon equipment qualification to verify the entire process.
This typically involves:
- Developing a detailed process validation protocol that specifies test conditions, sample sizes, acceptance criteria, and statistical methods
- Executing validation runs under normal operating conditions
- Collecting and analyzing data to demonstrate process consistency
- Documenting results in a validation report
Six Sigma practitioners bring statistical rigor to this stage by determining appropriate sample sizes, establishing meaningful acceptance criteria, and applying statistical tests to validation data.
Continued Process Verification
The validation journey doesn’t end once a process is qualified. Continued Process Verification ensures the process remains in a state of control throughout its commercial life.
Monitoring methods in this stage range from routine in-process checks to sophisticated statistical monitoring.
Key parameters identified during earlier stages undergo regular monitoring according to an established control plan.
The frequency and extent of monitoring should be based on risk assessment and process criticality.
Statistical process control (SPC) serves as the primary tool for ongoing monitoring. Control charts help detect process shifts before they result in quality problems. Different chart types (X-bar, R, EWMA, etc.) monitor different aspects of process performance.
Six Sigma teams select appropriate chart types based on process characteristics and the parameters being monitored.
When monitoring identifies potential issues, teams conduct investigations to determine root causes and implement corrective actions.
This investigation process should be formalized to ensure consistency and proper documentation.
Continuous improvement remains possible even with validated processes. Changes to improve efficiency or quality must follow formal change control procedures, with appropriate revalidation based on risk assessment.
Six Sigma’s DMAIC methodology provides a structured approach for implementing improvements while maintaining validated status.
Annual product reviews provide an opportunity to evaluate overall process performance and validation status.
These reviews examine trends across batches, investigate deviations, and assess whether the current validation strategy remains appropriate.
By implementing these three stages as part of a Six Sigma project, organizations create robust processes that consistently deliver quality products while satisfying regulatory requirements.
The integration of Six Sigma tools throughout the validation lifecycle enhances both efficiency and effectiveness.
Process Validation vs. Process Verification: Understanding the Difference
The terms “validation” and “verification” often cause confusion in regulated industries. While they sound similar and both relate to quality assurance, they serve distinct purposes in the manufacturing lifecycle.
Understanding the difference between process verification vs validation is crucial for regulatory compliance and effective quality management.
Process validation establishes documented evidence providing a high degree of assurance that a specific process will consistently produce a product meeting predetermined specifications and quality attributes. It’s proactive, forward-looking, and aims to prove future consistency.
Process verification, in contrast, confirms that specified requirements have been fulfilled for a specific instance or batch. It’s reactive, backward-looking, and confirms that a particular process run met its requirements.
This distinction might seem subtle, but it has significant implications for how companies approach quality management. Validation asks, “Will this process consistently work in the future?” while verification asks, “Did this specific instance work correctly?“
The key distinctions extend beyond this basic definition:
Validation typically occurs before routine commercial manufacturing begins. It requires multiple runs to demonstrate consistency and establish statistical confidence in the process.
Verification happens during or after production of specific batches, often as part of batch release testing.
Validation examines the entire process holistically, considering how different parameters interact and affect quality attributes. Verification often focuses on specific aspects or outputs of a process, such as testing finished products against specifications.
Validation relies heavily on statistical analysis to prove consistency across multiple runs. Six Sigma Green Belt certification programs teach many of these statistical tools, including process capability analysis and statistical tolerance intervals.
Verification might use simpler pass/fail criteria for specific attributes.
Validation documentation includes protocols that outline test conditions, acceptance criteria, and statistical methods, followed by reports summarizing results and conclusions.
Verification documentation typically includes test results, certificates of analysis, or inspection reports for specific batches.
When should you use each approach? The answer depends on several factors:
Use process validation when:
- Introducing new products or processes
- Making significant changes to existing processes
- Regulatory requirements mandate validation (most pharmaceutical and medical device manufacturing)
- Process failures could significantly impact patient safety
- You need to demonstrate long-term process consistency
Use process verification when:
- Confirming a specific batch meets requirements
- Performing routine quality control
- Investigating deviations or non-conformances
- Conducting periodic reviews of validated processes
- Supplementing validation with ongoing checks
From a compliance perspective, regulatory bodies have distinct expectations for validation and verification activities.
The FDA and other regulatory authorities require formal process validation for most pharmaceutical and medical device manufacturing processes.
These requirements appear in regulations like 21 CFR Parts 210/211 for pharmaceuticals and 21 CFR Part 820 for medical devices.
Verification activities, while important, generally don’t satisfy these validation requirements. Companies sometimes make the mistake of relying solely on verification (testing finished products) without proper validation (proving the process itself works consistently).
This approach has resulted in numerous FDA warning letters and regulatory actions.
The relationship between validation and verification isn’t either/or—they work together in a well-designed quality system.
Validation provides confidence that a process will perform consistently, while verification confirms that specific instances did perform as expected. Both are necessary for a robust quality assurance program.
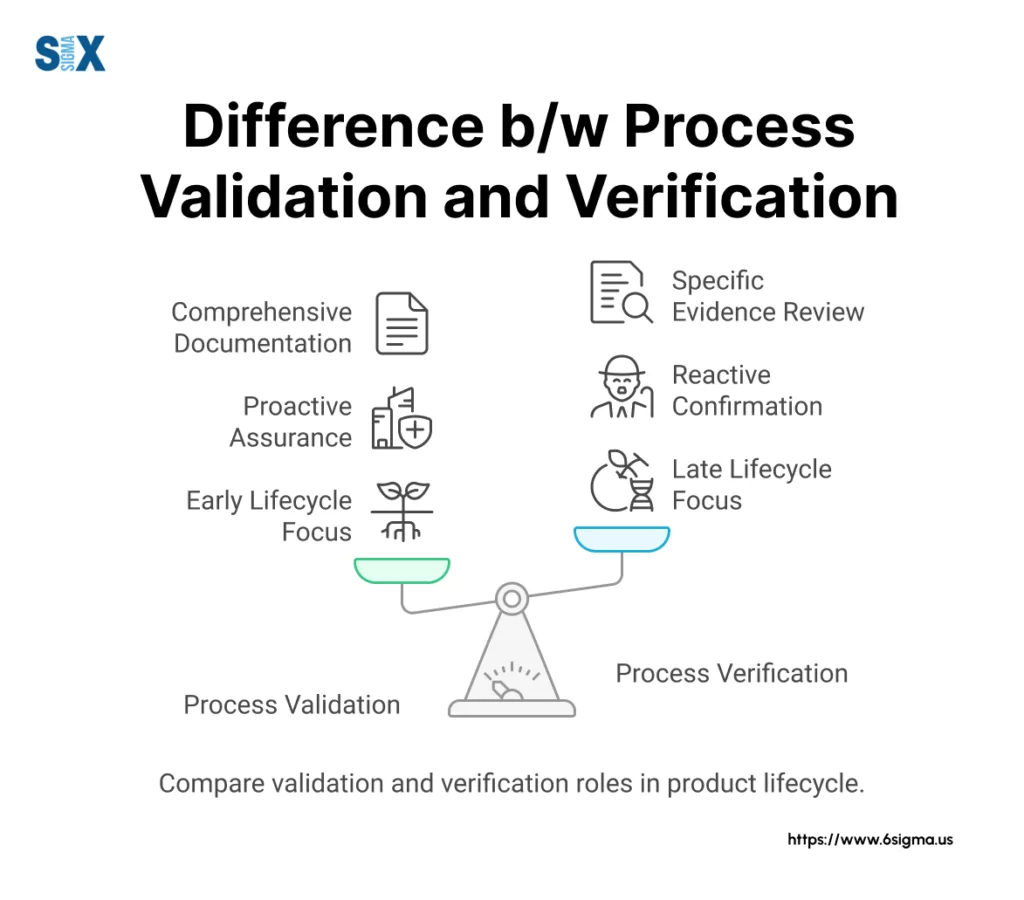
Many companies implement a lifecycle approach that integrates both validation and verification. Initial process validation establishes confidence in the process, while ongoing verification through monitoring and testing confirms the process remains in a validated state.
When verification identifies potential issues, companies can investigate and determine whether revalidation is necessary.
By understanding the distinct roles of validation and verification, quality professionals can build more effective quality systems that satisfy both regulatory requirements and business needs.
Best Practices for Process Validation in Six Sigma Projects
Successful process validation requires more than just following regulatory guidelines—it demands strategic planning, cross-functional collaboration, and statistical rigor.
When integrated with Six Sigma methodology, process validation becomes a powerful driver for both compliance and operational excellence.
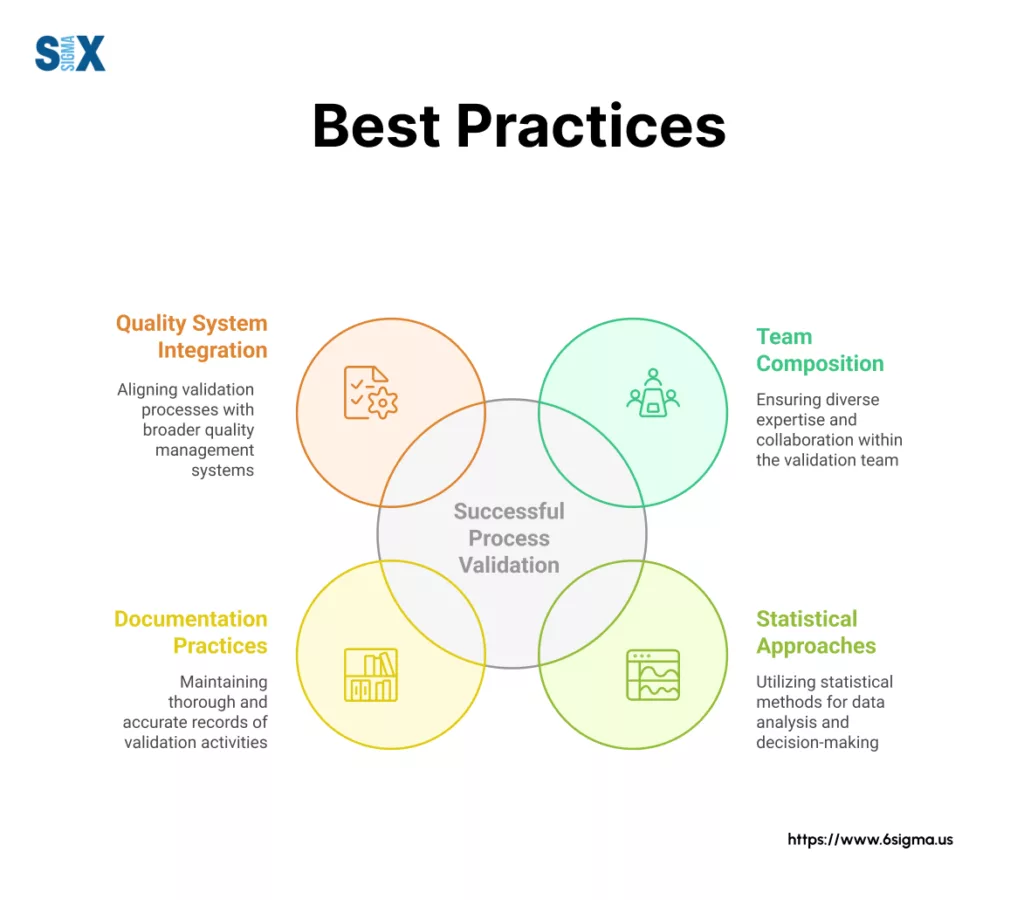
Effective validation teams bring together diverse expertise from across the organization. While specific roles vary by company, most successful teams include:
Quality Assurance specialists who understand regulatory requirements and documentation standards.
Process engineers who possess deep knowledge of the manufacturing process and its critical parameters.
Statistical experts, often holding Six Sigma Black Belt certification, who design validation studies and analyze results.
Regulatory affairs professionals who ensure validation activities satisfy current regulatory expectations.
Manufacturing representatives who provide practical insights about process operation and variability.
IT/automation specialists who support data collection and analysis systems.
The validation team should establish clear responsibilities for protocol development, execution, data analysis, and report generation. Documenting these responsibilities in a validation master plan helps prevent confusion and ensures accountability throughout the project.
Even well-planned validation projects face challenges. Common obstacles include:
Insufficient process understanding leads to validation studies that miss critical parameters or interactions. Solution: Conduct thorough process characterization studies before finalizing validation protocols.
Use Design of Experiments (DOE) to efficiently explore parameter interactions.
Inadequate statistical approaches result in validation studies that lack proper sample sizes or appropriate acceptance criteria. Solution:
Apply statistical power analysis to determine sample sizes and develop statistically valid acceptance criteria based on process capability requirements.
Poor documentation practices create compliance risks during regulatory inspections. Solution: Implement standardized templates for protocols and reports, with built-in quality checks to ensure completeness.
Time pressure sometimes leads to shortcuts in validation activities. Solution: Develop realistic timelines that account for potential issues, and use risk assessments to focus validation efforts on the most critical aspects.
Equipment or process changes during validation invalidate results and require rework.
Solution: Implement strict change control during validation studies, and consider qualification of equipment before beginning process validation.
Cross-site validation challenges arise when transferring processes between facilities.
Solution: Develop detailed transfer protocols that specify exactly what must be revalidated at the receiving site, based on risk assessment.
Several key success factors distinguish exceptional validation programs:
Risk-based approaches focus validation efforts where they matter most. By systematically identifying and evaluating risks, teams can develop validation strategies proportional to the level of risk.
This approach satisfies regulatory expectations while optimizing resource utilization.
Statistical thinking drives validation design and analysis. Rather than simply collecting data, successful teams use statistical methods to determine sample sizes, establish meaningful acceptance criteria, and analyze results with appropriate statistical tests.
Knowledge management systems capture insights from validation activities for future use.
These systems document not just what was done, but why specific approaches were chosen and what was learned during validation.
Continuous improvement mindset views validation as an opportunity to enhance processes, not just document them.
By applying Six Sigma tools throughout validation, teams can identify opportunities to reduce variability and improve efficiency.
Integration with quality systems ensures validation activities connect seamlessly with other quality processes like change control, deviation management, and annual product reviews.
Future Trends in Process Validation
Process validation continues to evolve as new technologies and regulatory approaches emerge. Several key trends are reshaping how companies approach validation in regulated industries.
Digital transformation is revolutionizing process validation through advanced data analytics and modeling.
Traditional validation relied heavily on physical testing, but modern approaches increasingly incorporate:
- Predictive modeling that simulates process performance under various conditions, reducing the need for extensive physical testing.
- Digital twins that create virtual replicas of physical processes, allowing teams to test changes virtually before implementing them physically.
- Real-time process monitoring systems that continuously collect and analyze data, enabling faster detection of process drift.
- Advanced data analytics that identify patterns and relationships not apparent through traditional analysis methods.
These digital tools don’t replace physical validation but complement it by providing deeper insights and focusing physical testing where it adds the most value.
These automation tools not only improve efficiency but also enhance compliance by ensuring consistent execution of validation activities and maintaining data integrity throughout the process.
Continuous manufacturing represents perhaps the most significant shift in process validation approaches.
Regulatory expectations continue to evolve toward more flexible, risk-based approaches.
Recent guidance from regulatory agencies emphasizes:
- Quality by Design (QbD) principles that build quality into processes through scientific understanding.
- Established Conditions that distinguish between critical aspects requiring regulatory oversight and less critical elements managed through internal change control.
- Continued Process Verification that maintains the validated state through ongoing monitoring rather than periodic re-validation.
These evolving regulatory approaches create opportunities for more efficient validation strategies focused on critical aspects that impact product quality and patient safety.
Companies that embrace these trends position themselves for both regulatory success and operational excellence.
By integrating Six Sigma principles with modern validation approaches, organizations can build robust processes that consistently deliver quality products while adapting to changing technologies and regulatory expectations.
SixSigma.us offers both Live Virtual classes as well as Online Self-Paced training. Most option includes access to the same great Master Black Belt instructors that teach our World Class in-person sessions. Sign-up today!
Virtual Classroom Training Programs Self-Paced Online Training Programs






